The New Drug Application (NDA) is the vehicle through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing in the United States.
The purpose of a NDA is to provide enough information to permit the FDA to reach the following key decisions
- Whether the drug is safe and effective in its proposed use(s), and whether the benefits of the drug outweigh the risks
- Whether the drug’s proposed labeling (package insert) is appropriate and what it should contain
- Whether the methods used in manufacturing the drug and the controls used to maintain the drug’s quality are adequate to preserve the drug’s identity, strength, quality, and purity
For more information on new drug applications, please visit the FDA’s How drugs are developed and approved page.
NRX-100 (ketamine)
Company: NRx Pharmaceuticals, Inc.
Treatment for: Major Depressive Disorder with Acute Suicidal Ideation
NRX-100 (ketamine) is an intravenous formulation of ketamine in development for the treatment of suicidal depression.
- NRx Pharmaceuticals, Inc. Files Initial Section of U.S. New Drug Application to the FDA for NRX-100 (IV Ketamine) for the Treatment of Suicidal Depression – December 30, 2024
See also: Generic approvals, New drug approvals, Recent additions to Drugs.com, Alphabetical listing of all new drug applications, FDA approval process
Avutometinib
Company: Verastem Oncology
Treatment for: Ovarian Cancer
Avutometinib is a RAF/MEK clamp in development for use in combination with defactinib for the treatment of adults with recurrent KRAS mutant low-grade serous ovarian cancer.
- Verastem Oncology Announces FDA Acceptance and Priority Review of New Drug Application for Avutometinib in Combination with Defactinib for the Treatment of Recurrent KRAS Mutant Low-Grade Serous Ovarian Cancer – December 30, 2024
- Verastem Oncology Completes Rolling NDA Submission to the FDA for Avutometinib Plus Defactinib as a Treatment for Recurrent KRAS Mutant Low-Grade Serous Ovarian Cancer – October 31, 2024
- Verastem Oncology Announces the Initiation of a Rolling Submission of NDA to FDA Seeking Accelerated Approval of Avutometinib and Defactinib Combination for the Treatment of Adult Patients with Recurrent KRAS Mutant Low-Grade Serous Ovarian Cancer – May 24, 2024
Sunvozertinib
Company: Dizal
Treatment for: Non Small Cell Lung Cancer
Sunvozertinib is an irreversible EGFR inhibitor in development for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) exon 20 insertion (exon20ins) mutations.
- U.S. FDA Granted Priority Review to Dizal’s Sunvozertinib New Drug Application – January 7, 2025
- Dizal Submits New Drug Application to the U.S. FDA for Sunvozertinib in Treating Relapsed or Refractory Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations – November 8, 2024
Ebvallo (tabelecleucel)
Company: Atara Biotherapeutics, Inc.
Treatment for: EBV-Positive Post-Transplant Lymphoproliferative Disease
Tabelecleucel (tab-cel) is an allogeneic, EBV-specific T-cell immunotherapy in development for the treatment of patients two years of age and older with Epstein-Barr virus positive post-transplant lymphoproliferative disease who have received at least one prior therapy.
- Atara Biotherapeutics Provides Regulatory and Business Update on Ebvallo (tabelecleucel) – January 16, 2025
- Atara Biotherapeutics Announces U.S. FDA Acceptance and Priority Review of the Biologics License Application for Tabelecleucel (Tab-cel®) for the Treatment of Epstein-Barr Virus Positive Post-Transplant Lymphoproliferative Disease – July 17, 2024
- Atara Biotherapeutics Submits Tabelecleucel (Tab-cel®) Biologics License Application for Treatment of Epstein-Barr Virus Positive Post-Transplant Lymphoproliferative Disease with U.S. FDA – May 20, 2024
Plozasiran
Company: Arrowhead Pharmaceuticals, Inc.
Treatment for: Familial Chylomicronemia Syndrome
Plozasiran is a first-in-class investigational RNA interference (RNAi) therapeutic in development for the treatment of familial chylomicronemia syndrome.
- Arrowhead Pharmaceuticals Announces Acceptance of New Drug Application by U.S. FDA of Plozasiran for the Treatment of Familial Chylomicronemia Syndrome – January 17, 2025
- Arrowhead Pharmaceuticals Submits New Drug Application to U.S. FDA for Plozasiran for the Treatment of Familial Chylomicronemia Syndrome – November 18, 2024
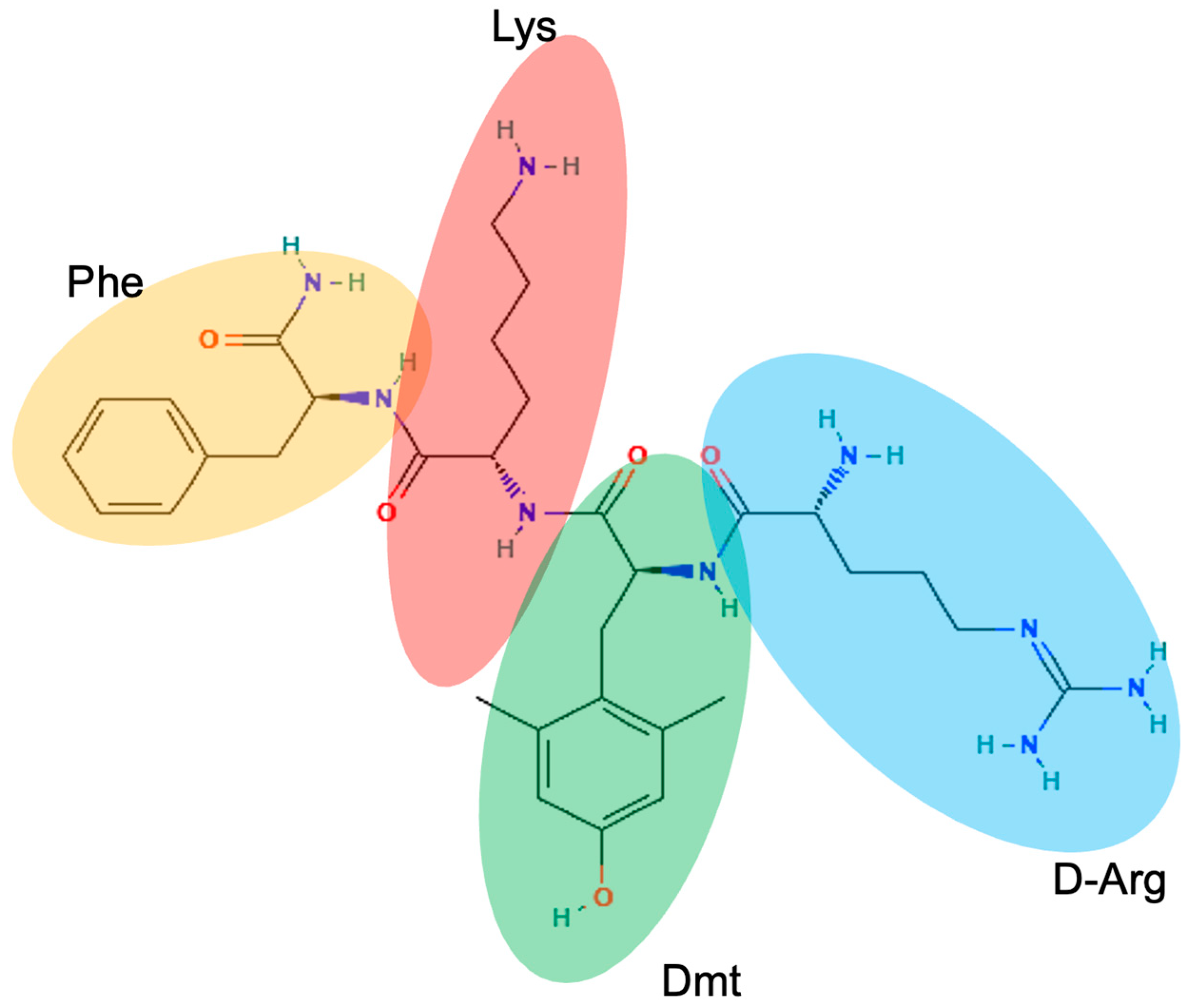
Elamipretide
Company: Stealth BioTherapeutics Inc.
Treatment for: Barth Syndrome
Elamipretide is a novel mitochondrial tetrapeptide in development for the treatment of Barth syndrome.
- Stealth BioTherapeutics Announces PDUFA Action Date Extension for Elamipretide to Treat Patients with Barth Syndrome – January 23, 2025
- Stealth BioTherapeutics Announces Positive Vote from FDA Advisory Committee Meeting Supporting Potential Approval of Elamipretide for the Treatment of Barth Syndrome – October 11, 2024
- Stealth Biotherapeutics Announces FDA Acceptance of New Drug Application for Elamipretide for the Treatment of Barth Syndrome – April 8, 2024
- Stealth BioTherapeutics Submits Elamipretide New Drug Application to FDA for Treatment of Barth Syndrome – August 24, 2021
SL1009 (sodium dichloroacetate) Oral Solution
Company: Saol Therapeutics
Treatment for: Pyruvate Dehydrogenase Complex Deficiency
SL1009 (sodium dichloroacetate) is a pan- pyruvate dehydrogenase kinase (PDK) inhibitor in development for the treatment of Pyruvate Dehydrogenase Complex Deficiency.
- Saol Therapeutics Announces FDA Acceptance of New Drug Application for SL1009 for Treatment of Pyruvate Dehydrogenase Complex Deficiency – January 28, 2025
- Saol Therapeutics Announces Submission of New Drug Application (NDA) to the U.S. FDA for SL1009 – December 3, 2024
Apitegromab
Company: Scholar Rock
Treatment for: Spinal Muscular Atrophy
Apitegromab is an investigational muscle-targeted therapy intended to improve motor function in people living with spinal muscular atrophy (SMA) who have been treated with certain existing SMA therapies.
- Scholar Rock Submits Biologics License Application (BLA) to the U.S. FDA for Apitegromab as a Treatment for Patients with Spinal Muscular Atrophy (SMA) – January 29, 2025
ET-400 (hydrocortisone) Oral Solution
Company: Eton Pharmaceuticals, Inc.
Treatment for: Adrenocortical Insufficiency
ET-400 (hydrocortisone) is a proprietary, room temperature stable, oral solution formulation of the approved glucocorticoid hydrocortisone in development for use in children.
- Eton Pharmaceuticals Announces Extension of PDUFA Goal Date for ET-400 – February 6, 2025
- Eton Pharmaceuticals Announces FDA Acceptance of New Drug Application for ET-400 (Hydrocortisone Oral Solution) – July 15, 2024
- Eton Pharmaceuticals Announces Submission to FDA of New Drug Application for ET-400 (Hydrocortisone Oral Solution) – April 30, 2024
Related Products
-
Ivermectin 12MG
$2.00 / Per Pill
-
Prednisone 10MG
$0.80 / Per Pill
-
Doxycycline 100MG
$2.00 / Per Pill