Experimental Drug
A new experimental drug is showing promise in slowing the progression of disability in individuals with multiple sclerosis (MS), according to recent findings from a large clinical trial. The results have sparked optimism among patients, neurologists, and researchers alike, as current treatment options for MS often focus primarily on managing symptoms rather than significantly altering the course of the disease.
A leading medical research expert has issued a stark warning that proposed federal funding cuts could have devastating consequences for the future of medical innovation, disease treatment, and public health advancements in the United States. The cuts, which would slash billions of dollars from research agencies such as the National Institutes of Health (NIH) and the Centers for Disease Control and Prevention (CDC), could bring critical research to a grinding halt and stall decades of progress in the biomedical field.
Multiple sclerosis
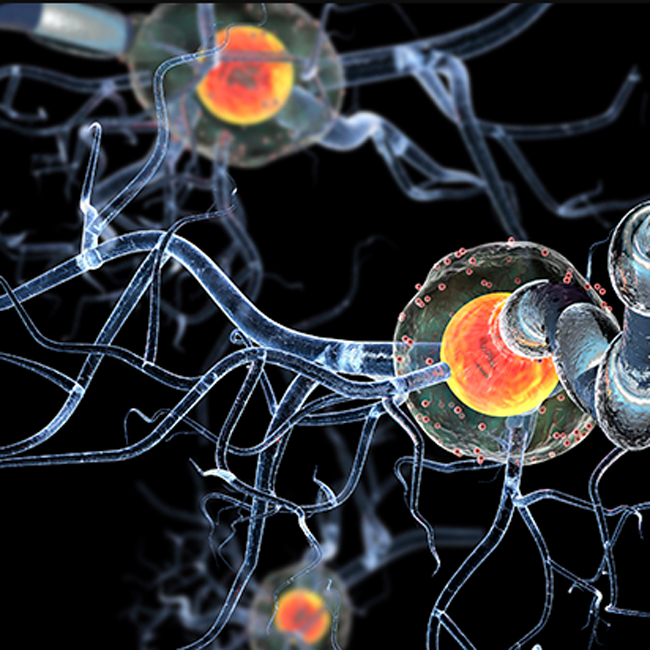
Multiple sclerosis is a chronic and often debilitating neurological condition that affects the central nervous system. It occurs when the immune system mistakenly attacks the protective covering of nerve fibers, known as myelin, leading to communication problems between the brain and the rest of the body. Over time, MS can cause physical and cognitive disability, with symptoms ranging from fatigue and muscle weakness to vision problems and difficulties with coordination and balance.
Experimental Medication
The experimental medication, whose name has not yet been released publicly due to its ongoing trial phase, targets specific pathways involved in the inflammatory and degenerative processes of MS. Unlike many current treatments that mainly address relapses or flare-ups of the disease, this drug appears to influence the underlying mechanisms that contribute to long-term disability.
In a randomized, double-blind, placebo-controlled phase 3 trial involving over 1,000 participants with progressive forms of MS, patients who received the experimental drug showed a statistically significant reduction in the rate of disability progression over a 24-month period. The findings were measured using widely accepted scales such as the Expanded Disability Status Scale (EDSS), which assesses walking ability, coordination, and other physical functions.

Dr. Lisa Grant, a neurologist and lead investigator in the study, described the results as “encouraging and potentially game-changing.” She explained that the drug not only reduced the pace at which disability developed but also appeared to preserve some cognitive function in participants—an area that is often neglected in MS treatments.
Therapy with such clear benefits
“This is the first time we’ve seen a therapy with such clear benefits for slowing long-term physical decline in patients with progressive MS,” Dr. Grant said. “It offers hope for a better quality of life, especially for those in whom other therapies have failed.”

Drug is Still Under Investigation
While the drug is still under investigation and requires further testing to confirm its safety and long-term efficacy, its development marks a significant milestone. Many existing MS therapies are focused on relapsing-remitting MS, a form of the disease characterized by intermittent flare-ups followed by periods of remission. Fewer options exist for those with primary or secondary progressive MS, where disability steadily worsens over time.
Some patients who participated in the trial have already reported noticeable improvements in their daily lives. “Before the study, I was having trouble walking even short distances,” said Anna Morales, a 42-year-old participant. “Since starting the drug, I’ve been able to move more freely and even return to light exercise. It’s made a huge difference.”
The drug’s manufacturer plans to submit the trial data to regulatory agencies later this year, with the hope of gaining approval for wider use. In the meantime, researchers continue to monitor participants for any long-term side effects and to determine if the benefits persist beyond the initial treatment window.
Experts caution that while the results are promising, more work is needed before the drug becomes widely available. Still, the study represents a significant leap forward in MS research and could pave the way for a new generation of therapies aimed at not just managing symptoms, but altering the course of the disease.
If approved, this drug could become a cornerstone treatment for progressive MS and offer new hope to the hundreds of thousands of individuals living with the disease worldwide.
Our Products
-
Lisinopril-20mg
$1.00 / Per Pill
-
Testosterone Enanthate
$240.00 / Per 10ml
-
Metformin 500MG
$0.80 / Per Pill