Oxidation and Disinfection: An Overview
Oxidation and disinfection are two critical processes widely used in water treatment, environmental science, industrial applications, and healthcare. Though distinct in purpose, these processes often overlap, especially in systems that require both the breakdown of contaminants and the elimination of harmful microorganisms.
Oxidation
Oxidation is a chemical reaction where a substance loses electrons, often involving the addition of oxygen or the removal of hydrogen. It plays a central role in environmental and industrial chemistry. In water treatment, oxidation is primarily used to transform harmful or undesirable contaminants into less harmful substances. Common targets for oxidation include iron, manganese, hydrogen sulfide, and various organic pollutants.
Oxidation can be achieved through different means:
Chemical oxidants like chlorine, ozone, hydrogen peroxide, or potassium permanganate.
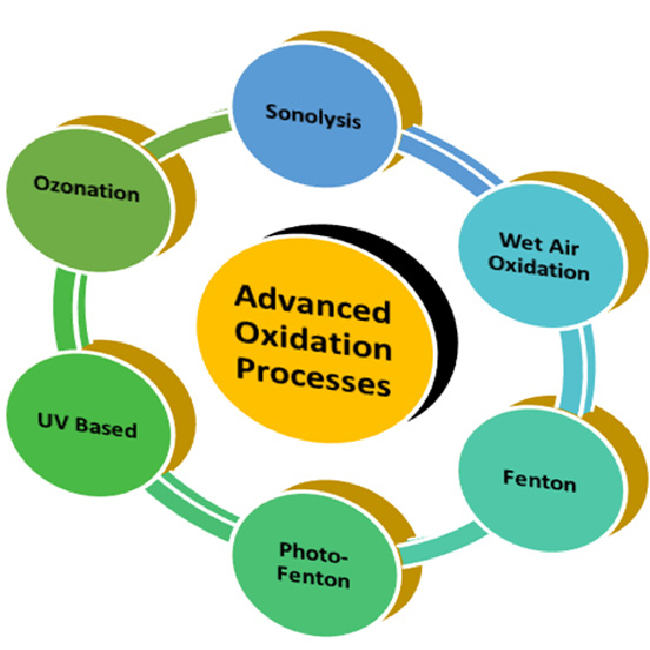
Advanced oxidation processes (AOPs) which combine oxidants with catalysts or ultraviolet (UV) light to produce hydroxyl radicals, highly reactive species capable of degrading complex organic molecules.
For example, in drinking water treatment, ozone and hydrogen peroxide are used to oxidize pesticides, pharmaceuticals, and other micropollutants that conventional treatment methods may not effectively remove. Oxidation also helps in reducing color, odor, and taste issues caused by organic matter.
Disinfection
Disinfection refers to the process of eliminating or inactivating pathogenic microorganisms to prevent disease transmission. Unlike sterilization, which aims to destroy all microbial life, disinfection focuses on reducing harmful organisms to safe levels. It is an essential step in water treatment, food processing, healthcare facilities, and public hygiene.
Common disinfectants include:
Chlorine and chloramine: Widely used due to their effectiveness and residual disinfection ability, though they can form disinfection byproducts (DBPs).
Ozone: A powerful disinfectant that leaves no chemical residue, but does not provide lasting protection.
Ultraviolet (UV) light: Physically inactivates microbes by damaging their DNA, effective against bacteria, viruses, and protozoa.
Hydrogen peroxide and peracetic acid: Used in hospital and industrial settings for surface disinfection.
In water treatment, disinfection is typically the final step after filtration and oxidation. Its primary goal is to inactivate pathogens like E. coli, Giardia, Cryptosporidium, and viruses that may pose health risks. Maintaining a disinfectant residual in distribution systems ensures ongoing protection as water travels through pipes to consumers.

Interplay Between Oxidation and Disinfection
Oxidation and disinfection often work hand-in-hand. Many chemical disinfectants are also oxidants. For instance, chlorine and ozone serve dual purposes by oxidizing contaminants and disinfecting water. Their oxidative properties allow them to break down cell walls, proteins, and nucleic acids of microorganisms, leading to cell death or inactivation.
Moreover, pre-oxidation can enhance disinfection efficiency. By breaking down organic matter and other interfering substances, oxidation can reduce the disinfectant demand and allow more of the disinfectant to target pathogens. Advanced oxidation processes (AOPs), which generate hydroxyl radicals, have gained attention for their ability to degrade persistent organic pollutants while simultaneously disinfecting water.
Applications and Importance
The importance of oxidation and disinfection extends far beyond water treatment:
In healthcare, disinfectants are used to sanitize medical instruments, surfaces, and air.
In food processing, oxidation helps in reducing spoilage and extending shelf life, while disinfection ensures safety against pathogens.
In industrial settings, oxidation can remove pollutants from wastewater, reducing environmental impact.
In air purification, ozone and UV systems are employed to neutralize airborne bacteria, viruses, and volatile organic compounds.
Conclusion
Oxidation and disinfection are foundational processes that contribute significantly to public health, environmental protection, and industrial efficiency. Their combined use allows for effective control of both chemical and biological contaminants. As concerns about emerging pathogens and chemical pollutants continue to grow, advancements in oxidation and disinfection technologies will play an increasingly vital role in ensuring clean water, safe environments, and healthier lives.
Our Products
-
Amoxicillin Capsules 500MG
$1.25 / Per Pill
-
Azithryomycin 500MG
$2.50 / Per Pill
-
Viagra 200MG
$1.50 / Per Pill